Description
Philips HeartStart OnSite/HS1 Defibrillator
Specifications
-
Battery Type - 9 volt DC, 4.2 Ah, composed of disposable long-life lithium manganese dioxide primary cells
Capacity - Minimum 200 shocks or 4 hours of operating time (EN60601-2-4:2003)
Install-by Date - Battery is labeled with an install-by date of at least five years from date of manufacture
Standby Life - Four years typical when battery is installed by the install-by date. (Will power the AED in standby state within the specified Standby Temperature range, assuming one battery insertion test and no defibrillation uses)
-
-
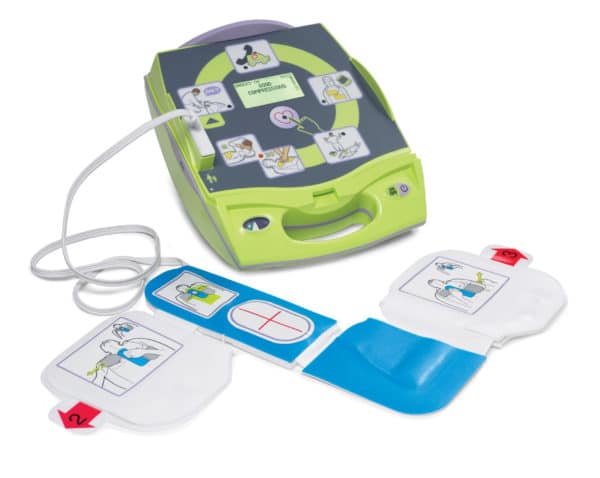
Product Specifications Kit Contents - Defibrillator, battery (one, pre-installed), SMART Pads (one set, pre-installed), set-up/maintenance guide with expiration date tags, owner’s manual, quick reference guide
Waveform - SMART Truncated exponential biphasic. Waveform parameters adjusted as a function of patient impedance
Defibrillation peak current – Adult - 32 A (150 J nominal) into a 50 ohm load
Defibrillation peak current – Pediatric - 19 A (50 J nominal) into a 50 ohm load (using optional infant/child SMART Pads Cartridge)
Shock-to-Shock Cycle Time - Typically less than 20 seconds between shocks in a series
Quick Shock - Able to deliver a shock after the end of a CPR interval, typically in eight seconds
Voice Instructions - Detailed voice messages guide responder through use of the defibrillator
CPR Guidance - Instructions for adult and infant/child CPR available at user selection
Shock Delivery - Via adhesive pads placed on patient’s bare skin as illustrated on pads
Controls - Green SMART Pads cartridge handle, green On/Off button, blue i-button, orange Shock button
Indicators - Ready light, blue i-button, caution light
-
-
SMART Pads Energy delivered - Adult: nominal 150 joules into a 50 ohm load; Infant/Child: nominal 50 joules into a 50 ohm load
How Supplied - Disposable cartridge, containing adhesive defibrillation pads, clicks into defibrillator for an integrated pads solution
Active Surface Area - 85 cm² (13.2 in²) each
Cable Length - Adult pads: 137 cm (54″); Infant/Child pads: 102 cm (40″)
Use-by Date - Cartridge is labeled with a use-by date of at least two years from date of manufacture
Training pads (optional) - Special pads put HeartStart into training mode and disable its energy delivery capability. Training pads feature 8 real-world training scripts. Use with training mat (included) or with adapters on manikins
-
-
Automated and User-activated Self-tests Daily Automatic Self-tests - Tests internal circuitry, waveform delivery system, pads cartridge, and battery capacity
Pads Integrity Test - Specifically tests readiness-for-use of pads (gel moisture)
Battery Insertion Test - Upon battery insertion, extensive automatic self-tests and user-interactive tests check device readiness
Status Indicator - Blinking green “Ready” light indicates ready for use. Audible “chirp” indicates need for maintenance
-
-
Data Recording and Transmission Infrared - Wireless transmission of event data to a PC or Palm PDA, using the IrDA protocol
Data Stored - First 15 minutes of ECG and the entire incident’s events and analysis decisions
-
-
Environmental/Physical Requirements Sealing - Solid objects per EN60529 class IP2X; Drip-proof per EN60529 class IPX1
Operating Temperature - 0 – 50 °C (32 – 122 °F)
Standby Temperature - 0 – 43 °C (32 – 109 °F)
Operating Altitude - 0 to 4,500 m (0 to 15,000 ft)
Operating Humidity - 0 to 95 % relative humidity (non-condensing)
Shock/Drop Abuse Tolerance - Withstands 1 meter drop to any edge, corner, or surface
Vibration - Meets EN1789 random and swept sine, road ambulance specification in operating and standby states
Standby Humidity - 0 to 75 % relative humidity (non-condensing)
Standby Altitude - > 48 hours from 0 to 8,500 ft and <48 hours from 8,500 to 15,000 ft
EMI (Radiated/Immunity) - Meets EN55011 Group 1 Level B Class B and EN61000-4-3
-